
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


22.6: Assigning Oxidation Numbers
- Last updated
- Save as PDF
- Page ID 53960
Moving from studying the element iron to iron compounds, we need to be able to clearly designate the form of the iron ion. An example of this is iron that has been oxidized to form iron oxide during the process of rusting. Although Antoine Lavoisier first began the idea of oxidation as a concept, it was Wendell Latimer (1893-1955) who gave us the modern concept of oxidation numbers. His 1938 book The Oxidation States of the Elements and Their Potentials in Aqueous Solution laid out the concept in detail. Latimer was a well-known chemist who later became a member of the National Academy of Sciences. Not bad for a gentleman who started college planning on being a lawyer.
Assigning Oxidation Numbers
The oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction. In oxidation-reduction processes, the driving force for chemical change is in the exchange of electrons between chemical species. A series of rules have been developed to determine oxidation numbers:
- For free elements (uncombined state), each atom has an oxidation number of zero. \(\ce{H_2}\), \(\ce{Br_2}\), \(\ce{Na}\), \(\ce{Be}\), \(\ce{K}\), \(\ce{O_2}\), \(\ce{P_4}\), all have an oxidation number of 0.
- Monatomic ions have oxidation numbers equal to their charge. \(\ce{Li^+} = +1\), \(\ce{Ba^{2+}} = +2\), \(\ce{Fe^{3+}} = +3\), \(\ce{I^-} = -1\), \(\ce{O^{2-}} = -2\), etc. Alkali metal oxidation numbers \(= +1\). Alkaline earth oxidation numbers \(= +2\). Aluminum \(= +3\) in all of its compounds. Oxygen's oxidation number \(= -2\) except when in hydrogen peroxide \(\left( \ce{H_2O_2} \right)\), or a peroxide ion \(\left( \ce{O_2^{2-}} \right)\) where it is \(-1\).
- Hydrogen's oxidation number is \(+1\), except for when bonded to metals as the hydride ion forming binary compounds. In \(\ce{LiH}\), \(\ce{NaH}\), and \(\ce{CaH_2}\), the oxidation number is \(-1\).
- Fluorine has an oxidation number of \(-1\) in all of its compounds.
- Halogens (\(\ce{Cl}\), \(\ce{Br}\), \(\ce{I}\)) have negative oxidation numbers when they form halide compounds. When combined with oxygen, they have positive numbers. In the chlorate ion \(\left( \ce{ClO_3^-} \right)\), the oxidation number of \(\ce{Cl}\) is \(+5\), and the oxidation number of \(\ce{O}\) is \(-2\).
- In a neutral atom or molecule, the sum of the oxidation numbers must be 0. In a polyatomic ion, the sum of the oxidation numbers of all the atoms in the ion must be equal to the charge on the ion.
Example \(\PageIndex{1}\)
What is the oxidation number for manganese in the compound potassium permanganate \(\left( \ce{KMnO_4} \right)\)?
The oxidation number for \(\ce{K}\) is \(+1\) (rule 2).
The oxidation number for \(\ce{O}\) is \(-2\) (rule 2).
Since this is a compound (there is no charge indicated on the molecule), the net charge on the molecule is zero (rule 6).
So we have:
\[\begin{align*} +1 + \ce{Mn} + 4 \left( -2 \right) &= 0 \\ \ce{Mn} - 7 &= 0 \\ \ce{Mn} &= +7 \end{align*}\nonumber \]
When dealing with oxidation numbers, we must always include the charge on the atom.
Another way to determine the oxidation number of \(\ce{Mn}\) in this compound is to recall that the permanganate anion \(\left( \ce{MnO_4^-} \right)\) has a charge of \(-1\). In this case:
\[\begin{align*} \ce{Mn} + 4 \left( -2 \right) &= -1 \\ \ce{Mn} - 8 &= -1 \\ \ce{Mn} &= +7 \end{align*}\nonumber \]
Example \(\PageIndex{2}\)
What is the oxidation number for iron in \(\ce{Fe_2O_3}\)?
\[\begin{align*} &\ce{O} \: \text{is} \: -2 \: \left( \text{rule 2} \right) \\ &2 \ce{Fe} + 3 \left( -2 \right) = 0 \\ &2 \ce{Fe} = 6 \\ &\ce{Fe} = 3 \end{align*}\nonumber \]
If we have the compound \(\ce{FeO}\), then \(\ce{Fe} + \left( -2 \right) = 0\) and \(\ce{Fe} = 2\). Iron is one of those materials that can have more than one oxidation number.
The halogens (except for fluorine) can also have more than one number. In the compound \(\ce{NaCl}\), we know that \(\ce{Na}\) is \(+1\), so \(\ce{Cl}\) must be \(-1\). But what about \(\ce{Cl}\) in \(\ce{NaClO_3}\)?
\[\begin{align*} \ce{Na} &= 1 \\ \ce{O} &= -2 \\ 1 + \ce{Cl} + 3 \left( -2 \right) &= 0 \\ 1 + \ce{Cl} - 6 &= 0 \\ \ce{Cl} - 5 &= 0 \\ \ce{Cl} &= +5 \end{align*}\nonumber \]
Not quite what we expected, but \(\ce{Cl}\), \(\ce{Br}\), and \(\ce{I}\) will exhibit multiple oxidation numbers in compounds.
- The oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction.
- In oxidation-reduction processes, the driving force for chemical change is in the exchange of electrons between chemical species.
- Six rules for determining oxidation numbers are listed.
- Examples of oxidation number determinations are provided.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
How to Assign Oxidation Numbers

The oxidation number is the positive or negative number of an atom that indicates the electrical charge the atom has if its compound consists of ions. In other words, the oxidation number gives the degree of oxidation (loss of electrons) or reduction (gain of electrons) of the atom in a compound. Because they track the number of electrons lost or gained, oxidation numbers are a sort of shorthand for balancing charge in chemical formulas.
This is a list of rules for assigning oxidation numbers, with examples showing the numbers for elements, compounds, and ions.
Rules for Assigning Oxidation Numbers
Various texts contain different numbers of rules and may change their order. Here is a list of oxidation number rules:
- Write the cation first in a chemical formula, followed by the anion. The cation is the more electropositive atom or ion, while the anion is the more electronegative atom or ion. Some atoms may be either the cation or anion, depending on the other elements in the compound. For example, in HCl, the H is H + , but in NaH, the H is H – .
- Write the oxidation number with the sign of the charge followed by its value. For example, write +1 and -3 rather than 1+ and 3-. The latter form is used to indicate oxidation state .
- The oxidation number of a free element or neutral molecule is 0. For example, the oxidation number of C, Ne, O 3 , N 2 , and Cl 2 is 0.
- The sum of all the oxidation numbers of the atoms in a neutral compound is 0. For example, in NaCl, the oxidation number of Na is +1, while the oxidation of Cl is -1. Added together, +1 + (-1) = 0.
- The oxidation number of a monatomic ion is the charge of the ion. For example, the oxidation number of Na + is +1, the oxidation number of Cl – is -1, and the oxidation number of N 3- is -3.
- The sum of the oxidation numbers of a polyatomic ion is the charge of the ion. For example, the sum of the oxidation numbers for SO 4 2- is -2.
- The oxidation number of a group 1 (alkali metal) element in a compound is +1.
- The oxidation number of a group 2 (alkaline earth) element in a compound is +2.
- The oxidation number of a group 7 (halogen) element in a compound is -1. The exception is when the halogen combines with an element with higher electronegativity (e.g., oxidation number of Cl is +1 in HOCl).
- The oxidation number of hydrogen in a compound is usually +1. The exception is when hydrogen bonds with metals forming the hydride anion (e.g., LiH, CaH 2 ), giving hydrogen an oxidation number of -1.
- The oxidation number of oxygen in a compound is usually -2. Exceptions include OF 2 and BaO 2 .
Examples of Assigning Oxidation Numbers
Example 1: Find the oxidation number of iron in Fe 2 O 3 .
The compound has no electrical charge, so the oxidation numbers of iron and oxygen balance each other out. From the rules, you know the oxidation number of oxygen is usually -2. So, find the iron charge that balances the oxygen charge. Remember, the total charge of each atom is its subscript multiplied by its oxidation number. O is -2 There are 3 O atoms in the compound so the total charge is 3 x -2 = -6 The net charge is zero (neutral), so: 2 Fe + 3(-2) = 0 2 Fe = 6 Fe = 3
Example 2: Find the oxidation number for Cl in NaClO3.
Usually, a halogen like Cl has an oxidation number of -1. But, if you assume Na (an alkali metal) has an oxidation number of +1 and O has an oxidation number of -2, the charges don’t balance out to give a neutral compound. It turns out all of the halogens, except for fluorine, have more than one oxidation number. Na = +1 O = -2 1 + Cl + 3(-2) = 0 1 + Cl -6 = 0 Cl -5 = 0 Cl = -5
- IUPAC (1997) “Oxidation Number”. Compendium of Chemical Terminology (the “Gold Book”) (2nd ed.). Blackwell Scientific Publications. doi: 10.1351/goldbook
- Karen, P.; McArdle, P.; Takats, J. (2016). “Comprehensive definition of oxidation state (IUPAC Recommendations 2016)”. Pure Appl. Chem . 88 (8): 831–839. doi: 10.1515/pac-2015-1204
- Whitten, K. W.; Galley, K. D.; Davis, R. E. (1992). General Chemistry (4th ed.). Saunders.
Related Posts
What Are the Rules for Assigning Oxidation Numbers?
Redox Reactions and Electrochemistry
andriano_cz / Getty Images
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Electrochemical reactions involve the transfer of electrons . Mass and charge are conserved when balancing these reactions, but you need to know which atoms are oxidized and which atoms are reduced during the reaction. Oxidation numbers are used to keep track of how many electrons are lost or gained by each atom. These oxidation numbers are assigned using the following rules.
Rules for Assigning Oxidation Numbers
- The convention is that the cation is written first in a formula, followed by the anion . For example, in NaH, the H is H-; in HCl, the H is H+.
- The oxidation number of a free element is always 0. The atoms in He and N 2 , for example, have oxidation numbers of 0.
- The oxidation number of a monatomic ion equals the charge of the ion. For example, the oxidation number of Na + is +1; the oxidation number of N 3- is -3.
- The usual oxidation number of hydrogen is +1. The oxidation number of hydrogen is -1 in compounds containing elements that are less electronegative than hydrogen, as in CaH 2 .
- The oxidation number of oxygen in compounds is usually -2. Exceptions include OF 2 because F is more electronegative than O, and BaO 2 , due to the structure of the peroxide ion, which is [O-O] 2- .
- The oxidation number of a Group IA element in a compound is +1.
- The oxidation number of a Group IIA element in a compound is +2.
- The oxidation number of a Group VIIA element in a compound is -1, except when that element is combined with one having a higher electronegativity. The oxidation number of Cl is -1 in HCl, but the oxidation number of Cl is +1 in HOCl.
- The sum of the oxidation numbers of all of the atoms in a neutral compound is 0.
- The sum of the oxidation numbers in a polyatomic ion is equal to the charge of the ion. For example, the sum of the oxidation numbers for SO 4 2- is -2.
- Assigning Oxidation States Example Problem
- How to Balance Net Ionic Equations
- Balance Redox Reaction Example Problem
- The Difference Between Oxidation State and Oxidation Number
- 5 Steps for Balancing Chemical Equations
- Chemistry Vocabulary Terms You Should Know
- How to Neutralize a Base With an Acid
- Net Ionic Equation Definition
- Reduction Definition in Chemistry
- Learn About Redox Problems (Oxidation and Reduction)
- Oxidation Reduction Reactions—Redox Reactions
- Balanced Equation Definition and Examples
- Oxidation Definition and Example in Chemistry
- Types of Chemical Reactions
- Reactions in Water or Aqueous Solution
- Molecules and Moles in Chemistry
Chemistry Learner
It's all about chemistry.
- Chemical Bonds
- Chemical Reactions
- Materials Chemistry
- Organic Chemistry
- Periodic Trends
- Periodic Table Groups
- How to Read Periodic Table
- Naming Covalent Compounds Worksheets
- Net Ionic Equation Worksheets
- Types of Chemical Reactions Worksheets
- Word Equations Worksheets
- Valence Electrons Worksheets
- Graphing Periodic Trends Worksheets
- Periodic Trends Ionization Energy Worksheets
- Atomic Structure And Isotopes Worksheets
Oxidation Number (Oxidation State)
What is oxidation number, oxidation number rules [1-6], how to find oxidation number.
An oxidation number is a number that is assigned to an atom to indicate its state of oxidation or reduction during a chemical reaction. Each atom in a redox reaction is assigned an oxidation number to understand its ability to donate, accept, or share electrons. It shows the total number of electrons that have been removed from or added to an element to get to its present state. For example, in Fe 2 O 3 , the oxidation number of Fe is +3, and in FeO, it is +2. A loss of electrons corresponds to an increase in oxidation number. On the other hand, a gain of electrons corresponds to a decrease in oxidation number [1-4] .
In order to assign oxidation numbers to atoms, we need to follow a set of rules.
1. The oxidation number of an element in its free state is zero.
Example : The oxidation number of Zn, Al, H 2 , O 2 , and Cl 2 is zero
2. The oxidation number of a monatomic ion is the same as the charge on the ion.
Example : The oxidation number of Na + is +1, Mg 2+ is +2, Al 3+ is +3, Cl -1 is -1, and O 2- is -2.
3. The sum of all oxidation numbers in a neutral compound is zero. The sum of all oxidation numbers in a polyatomic ion is equal to the charge on the ion.
Example : In Fe 2 O 3 , the oxidation number of Fe is +3, and that of O is -2. The sum of all oxidation numbers is: +3 x 2 + (-2) x 3 = 0. The result is expected since Fe 2 O 3 is neutral.
4. The oxidation number of an alkali metal in a compound is +1, and the oxidation number of an alkaline earth metal in a compound is +2.
Example : In NaCl, Na is an alkali metal, and its oxidation number is +1. In MgO, Mg is an alkaline earth metal, and its oxidation number is +2.
5. The oxidation number of oxygen in a compound is usually -2. However, if the oxygen is in a category of compounds called peroxides, its oxidation number is -1. If the oxygen is bonded to fluorine, the number is +1 or +2, depending upon the compound.
Example : The oxidation number of O in H 2 O is -2, in H 2 O 2 is -1, in OF 2 is +2, and in O 2 F 2 is +1
6. The oxidation number of hydrogen in a compound is usually +1. In the case of a binary metal hydride, the oxidation number is -1.
Example : The oxidation number of H is +1 in H 2 O and -1 in NaH.
7. The oxidation number of fluorine is always -1.
Example : The oxidation number of F in NaF is -1.
8. Chlorine , bromine, and iodine usually have an oxidation number of -1 unless bonded to oxygen or fluorine.
Example : The oxidation number of Cl in NaCl is -1 and in ClO 2 is +4, and in FCl is +1.

The oxidation number of an atom in an ion or compound can be determined using the above rules. Let us look at a few examples [1-6] .
1. Sulfuric Acid (H 2 SO 4 )
The oxidation number of hydrogen (H) and oxygen (O) are +1 and -2, respectively. Sulfuric acid is a neutral compound. Let x be the oxidation number of sulfur (S). Therefore,
(+1) x 2 + x + (-2) x 4 = 0
Or, 2 + x – 8 = 0
2. Nitric Acid (HNO 3 )
The oxidation numbers of hydrogen (H) and oxygen (O) are +1 and -2, respectively. Nitric acid is a neutral compound. Let x be the oxidation number of nitrogen (N). Therefore,
+1 + x + (-2) x 3 = 0
Or, +1 + x – 6 = 0
3. Potassium Permanganate (KMnO 4 )
The oxidation numbers of potassium (K) is +1 and oxygen (O) is -2. KMnO 4 is a neutral compound. Let x be the oxidation number of magnesium (Mn). Therefore,
+1 + x + (-2) x 4 = 0
Or, +1 + x – 8 = 0
4. Dichromate Ion (Cr 2 O 7 2- )
Dichromate is a complex ion. The oxidation number of oxygen (O) is -2. The charge of Cr 2 O 7 2- is -2. Let x be the oxidation number of chromium (Cr).
2x + (-2) x 7 = -2
Or, 2x -14 = -2
5. Carbonate (CO 3 2- )
The oxidation number of oxygen (O) is -2 and the charge on CO 3 2- is -2. Let x be the oxidation number of carbon (O). Therefore,
x + (-2) x 3 = -2
Or, x – 6 = -2
6. Phosphite (PO 3 3- )
The oxidation number of oxygen (O) is -2 and the charge of PO 3 3- is -3. Let x be the oxidation number of phosphorous (P). Therefore,
x + (-2) x 3 = -3
Or, x – 6 = -3
7. Potassium Perchlorate (KClO 4 )
The oxidation numbers of potassium (K) and oxygen (O) are +1 and -2, respectively. Let x be the oxidation number of chlorine (Cl). KClO 4 is a neutral compound. Therefore,
Or, 1 + x – 8 = 0
8. Potassium Nitrate (KNO 3 )
The oxidation number of potassium (K) and oxygen (O) are +1 and -2, respectively. KNO 3 is a neutral compound. Let x be the oxidation number of nitrogen (N). Therefore,
Or, 1 + x – 6 = 0
The following image shows a chart consisting of the oxidation numbers of the periodic table elements [7].

Ans. The difference between valency and oxidation number is that valency is the maximum number of electrons an atom can donate, accept, or share to become stable. In contrast, the oxidation number is the number of electrons an atom can donate or accept to form a bond with another atom.
Ans. The d-block or translational elements have incomplete d- and s-subshells. The valence electrons are present in both these subshells. It is for this reason that they can form variable oxidation states.
- Chem.libretexts.org
- Chemistrytalk.org
- Khanacademy.org
- Mccord.cm.utexas.edu
- Courses.lumenlearning.com
- Chemguide.co.uk
- Chemed.chem.purdue.edu
Related Articles

Crystal Field Theory

Van der Waals Equation

Gibbs’ Phase Rule

Gay-Lussac’s Law

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Trending Topics
© 2024 ( Chemistry Learner )
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History
... and beyond
- Socratic Meta
- Featured Answers

- Oxidation Numbers
Key Questions
The valence electrons determine how many electrons an atom is willing to give up or how many spaces need to be filled in order to satisfy the rule of octet.
Lithium (Li), Sodium (Na) and Potassium (K) all have an electron configuration that ends as #s^1# . Each of these atoms would readily release this electron to have a filled valence shell and become stable as #Li^+1# , #Na^+1# and #K^+1# . Each element having an oxidation state of +1.
Oxygen (O) and Sulfur (S) all have an electron configuration that ends as #s^2 p^4# . Each of these atoms would readily take on two electrons to have a filled valence shell and become stable as #O^-2# , and #S^-2# . Each element having an oxidation state of -2.
There are exceptions to the rules and the transition metals usually have more than one oxidation state.
I hope this was helpful. SMARTERTEACHER

And so we can interpret a given chemical reaction with the use of oxidation numbers. Consider the oxidation of ammonia to give nitrate ion..........in terms of formal oxidation state this is the transition, #stackrel(-III)N# to #stackrel(+V)N# , an 8 electron oxidation, which we formally represent in the equation.......
#NH_3(aq) +3H_2O rarr NO_3^(-) +9H^(+) + 8e^(-)# #(i)#
Is this balanced with respect to mass and charge? It must be if we purport to represent physical reality.
And, inevitably, something must be reduced to effect the oxidation; let's say it is oxygen.
#stackrel(0)O_2+4e^(-) rarr2O^(2-)# #(ii)#
We add the individual redox equations together in a way to eliminate the electrons, which are particles of convenience......And so we take #(i) + 2xx(ii)# :
#NH_3(aq) +3H_2O +2O_2+8e^(-)rarr NO_3^(-) +underbrace(9H^(+) +4O^(2-))_(4H_2O+H^+) + 8e^(-)#
And so we cancel out what we can.....
#NH_3 +cancel(3H_2O) +2O_2+cancel(8e^(-))rarr NO_3^(-) +underbrace(9H^(+) +4O^(2-))_(cancel(4)H_2O+H^+) + cancel(8e^(-))#
....to give finally.........
#NH_3 +2O_2rarr NO_3^(-) +H_2O+H^+#
....or........
#stackrel(-III)NH_3 +2stackrel(0)O_2rarr Hstackrel(+V)NO_3 +H_2stackrel(-II)O#
I acknowledge that is a lot of pfaff.......but your question was rather open-ended.

Assigning Oxidation Numbers Based on Chemical Rules

- For example, Al (s) and Cl 2 both have oxidation numbers of 0 because they are in their uncombined elemental forms.
- Note that sulfur's elemental form, S 8 , or octasulfur, though irregular, also has an oxidation number of 0.

- For instance, the ion Cl - has an oxidation number of -1.
- The Cl ion still has an oxidation number of -1 when it's part of the compound NaCl. Because the Na + ion, by definition, has a charge of +1, we know that the Cl - ion has a charge of -1, so its oxidation number is still -1.

- For example, let's examine a compound containing the metallic aluminum ion. The compound AlCl 3 has an overall charge of 0. Because we know that Cl - ions have a charge of -1 and there are 3 Cl - ions in the compound, the Al ion must have a charge of +3 so that the overall charge of all the ions adds to 0. Thus, Al's oxidation number is +3 in this compound.

- When oxygen is in its elemental state (O 2 ), its oxidation number is 0, as is the case for all elemental atoms.
- When oxygen is part of a peroxide, its oxidation number is -1. Peroxides are a class of compounds that contain an oxygen-oxygen single bond (or the peroxide anion O 2 -2 ). For instance, in the molecule H 2 O 2 (hydrogen peroxide), oxygen has an oxidation number (and a charge) of -1.
- When oxygen is part of a superoxide , its oxidation number is -1⁄2. Superoxides contain the superoxide anion O 2 - .
- When oxygen is bound to fluorine, its oxidation number is +2. See fluorine rule below for more info. However, there is an exception: in (O 2 F 2 ), the oxidation number of oxygen is +1.

- For instance, in H 2 O, we know that hydrogen has an oxidation number of +1 because oxygen has a charge of -2 and we need two +1 charges to make the compound's charges add up to zero. However, in sodium hydride, NaH, hydrogen has an oxidation number of -1 because the Na + ion has a charge of +1 and, for the compound's total charge to equal zero, hydrogen's charge (and thus oxidation number) must equal -1.

- This is a good way to check your work - if the oxidation in your compounds don't add up to the charge of your compound, you know that you have assigned one or more incorrectly.
Assigning Numbers to Atoms Without Oxidation Number Rules

- For example, in the compound Na 2 SO 4 , the charge of sulfur (S) is unknown - it's not in its elemental form, so it's not 0, but that's all we know. This is a good candidate for this method of algebraic oxidation number determination.

- In Na 2 SO 4 , we know, based on our set of rules, that the Na ion has a charge (and thus oxidation number) of +1 and that the oxygen atoms have oxidation numbers of -2.

- In Na 2 SO 4 , we know there are 2 Na atoms and 4 O atoms. We would multiply 2 × +1, the oxidation number of Na, to get an answer of 2, and we would multiply 4 × -2, the oxidation number of O, to get an answer of -8.

- In our Na 2 SO 4 example, we would add 2 to -8 to get -6.

- (Sum of known oxidation numbers) + (unknown oxidation number you are solving for) = (charge of the compound)
- S = 6. S has an oxidation number of 6 in Na 2 SO 4 .
Community Q&A
- In a compound, the sum of all the oxidation numbers must equal 0. If there is an ion that has 2 atoms, for example, the sum of the oxidation numbers must equal the ionic charge. Thanks Helpful 3 Not Helpful 0
- It is very helpful to know how to read a periodic table of elements and where the metals and nonmetals are located. Thanks Helpful 2 Not Helpful 2
- Atoms in their elemental form always have an oxidation number of 0. A monatomic ion has an oxidation number equal to its charge. Group 1 metals in the elemental form, such as hydrogen, lithium and sodium, have an oxidation number of +1; group 2 metals in their elemental form, such as magnesium and calcium, have an oxidation number of +2. Both hydrogen and oxygen have a possibility of 2 different oxidation numbers depending on to what they are bonded. Thanks Helpful 2 Not Helpful 0

Things You'll Need
- Periodic table of elements
- Access to the internet, chemistry textbooks or both
- Paper, pen or pencil
You Might Also Like

Expert Interview

Thanks for reading our article! If you’d like to learn more about chemistry, check out our in-depth interview with Anne Schmidt .
- ↑ https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemistry/Definitions_of_Oxidation_and_Reduction
- ↑ https://byjus.com/chemistry/how-to-calculate-oxidation-number/
- ↑ https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch2/oxnumb.html
- ↑ https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemistry/Oxidation_States_(Oxidation_Numbers)
- ↑ https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/types-of-chemical-reactions/a/oxidation-number
- http://www.chemguide.co.uk/inorganic/redox/oxidnstates.html
About This Article

To find oxidation numbers, figure out if the substance in question is elemental or an ion. If it’s elemental, it has an oxidation number of 0. If it’s an ion, its oxidation number is the same as its charge. Be aware that metallic ions that can have more than one charge, like iron, can also have more than one oxidation number! In most cases assign an oxidation number of -2 to oxygen and +1 to hydrogen. In all cases give fluorine an oxidation number of -1. Finally, be sure the oxidation numbers in a compound are equal to the compound’s charge. For more information on finding oxidation numbers, including for atoms that don’t have oxidation number rules, read on! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Tanmay Srivastava
Jul 25, 2017
Did this article help you?
Ayesha Raza
Dec 12, 2017
Madison Kisling
Jun 1, 2017
May 26, 2020
Sushma Karanam
Jul 9, 2016

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Develop the tech skills you need for work and life

- Periodic table of the elements
- Chemistry calculators
- Image gallery
OXIDATION NUMBERS CALCULATOR
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'Calculate' (for example: Ca2+ , HF2^- , Fe4[Fe(CN)6]3 , NH4NO3 , so42- , ch3cooh , cuso4*5h2o ).
The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The oxidation number is synonymous with the oxidation state. Determining oxidation numbers from the Lewis structure (Figure 1a) is even easier than deducing it from the molecular formula (Figure 1b). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Bonds between atoms of the same element (homonuclear bonds) are always divided equally.
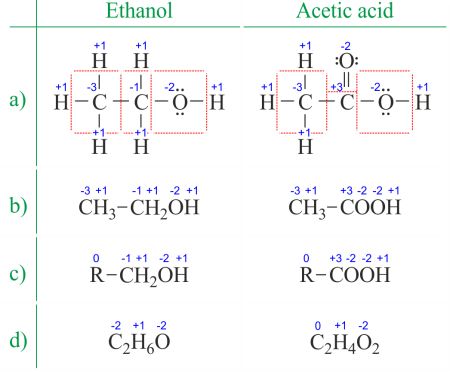
When dealing with organic compounds and formulas with multiple atoms of the same element, it's easier to work with molecular formulas and average oxidation numbers (Figure 1d). Organic compounds can be written in such a way that anything that doesn't change before the first C-C bond is replaced with the abbreviation R (Figure 1c). Unlike radicals in organic molecules, R cannot be hydrogen. Since the electrons between two carbon atoms are evenly spread, the R group does not change the oxidation number of the carbon atom it's attached to. You can find examples of usage on the Divide the redox reaction into two half-reactions page.
Rules for assigning oxidation numbers
- The oxidation number of a free element is always 0.
- The oxidation number of a monatomic ion equals the charge of the ion.
- Fluorine in compounds is always assigned an oxidation number of -1.
- The alkali metals (group I) always have an oxidation number of +1.
- The alkaline earth metals (group II) are always assigned an oxidation number of +2.
- Oxygen almost always has an oxidation number of -2, except in peroxides (H 2 O 2 ) where it is -1 and in compounds with fluorine (OF 2 ) where it is +2.
- Hydrogen has an oxidation number of +1 when combined with non-metals, but it has an oxidation number of -1 when combined with metals.
- The algebraic sum of the oxidation numbers of elements in a compound is zero.
- The algebraic sum of the oxidation states in an ion is equal to the charge on the ion.
Assigning oxidation numbers to organic compounds
- cysteine: HO2CCH(NH2)CH2SH
Back to top ↑
Citing this page:
Generalic, Eni. "Oxidation numbers calculator." EniG. Periodic Table of the Elements . KTF-Split, 18 Jan. 2024. Web. {Date of access} . <https://www.periodni.com/oxidation_numbers_calculator.php>.
- Online calculators
- Scientific calculator
- Preparation of solutions
- Oxidation numbers calculator
- Balancing redox reactions
- Memory game
- Articles and tables
- Chemistry dictionary
- Printable periodic table
- Portable applications
- Chemistry images gallery
- Short form of the periodic table
- Long form of the periodic table
- History of the Periodic table of elements
- Electronic configurations of the elements
- Alphabetical list of chemical elements
- Naming of elements of atomic numbers greater than 100
- ASCII Periodic table
- Scientific calculator for chemists
- Gas laws calculator
- Molar mass calculator
- Angle converter
- Roman numerals converter
- Number systems converter
- Labeling of chemical containers
- Oxidation number change method
- Ion-electron method
- Gauss elimination method
- Find the pairs
- List of abbreviations and acronyms
- Crystal systems and Bravais lattices
- GHS - Hazard pictograms
- NFPA 704 Hazard Diamond
- Fundamental physical constants
- Solubility product constants
- SI - International System of Units
- Composition of mixtures and solutions
- Stoichiometric calculations
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Climate change
- Global warming and mankind
- Story of ozone and ozone holes
- World War 3: Battle for Earth
- The ozone layer is not a shield
- Hexadecimal color codes
- Writing equations on the Web
- Writing chemical equations on the Web
- Character entity references in HTML
- Unicode UTF-8 encoding
- Download PDF Documents
- Download Software
- Download Images
- Periodic table
- Français
- Español
- Search on periodni.com

IMAGES
VIDEO
COMMENTS
Assigning Oxidation Numbers. The oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction. In oxidation-reduction processes, the driving force for chemical change is in the exchange of electrons between chemical species. A series of rules have been developed to determine ...
This chemistry tutorial discusses how to assign oxidation numbers and includes examples of how to determine the oxidation numbers in a compound following som...
Write the oxidation number with the sign of the charge followed by its value. For example, write +1 and -3 rather than 1+ and 3-. The latter form is used to indicate oxidation state. The oxidation number of a free element or neutral molecule is 0. For example, the oxidation number of C, Ne, O 3, N 2, and Cl 2 is 0.
Rules for Assigning Oxidation Numbers. The convention is that the cation is written first in a formula, followed by the anion. For example, in NaH, the H is H-; in HCl, the H is H+. The oxidation number of a free element is always 0. The atoms in He and N 2, for example, have oxidation numbers of 0. The oxidation number of a monatomic ion ...
Oxidation numbers are assigned to elements using these rules: Rule 1: The oxidation number of an element in its free (uncombined) state is zero — for example, Al (s) or Zn (s). This is also true for elements found in nature as diatomic (two-atom) elements. Rule 3: The sum of all oxidation numbers in a neutral compound is zero.
By assigning oxidation numbers to the atoms of each element in a redox equation, we can determine which element is oxidized and which element is reduced during the reaction. In this video, we'll use this method to identify the oxidized and reduced elements in the reaction that occurs between I⁻ and MnO₄⁻ in basic solution. Created by Sal ...
Oxygen's oxidation number is -2 except when in hydrogen peroxide (H 2 O 2 ), or a peroxide ion (O 22-) where it is -1. Hydrogen's oxidation number is +1, except for when bonded to metals as the hydride ion forming binary compounds. In LiH, NaH, and CaH 2, the oxidation number is -1. Fluorine has an oxidation number of -1 in all of its ...
In order to assign oxidation numbers to atoms, we need to follow a set of rules. 1. The oxidation number of an element in its free state is zero. Example: The oxidation number of Zn, Al, H 2, O 2, and Cl 2 is zero. 2. The oxidation number of a monatomic ion is the same as the charge on the ion.
Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Assigning these numbers involves several rules: Free atoms (H2) usually have an oxidation number of 0, monoatomic ions (Cl-) are usually equal to their charge, and polyatomic ions have several governing principles.
Let x equal the oxidation number for sulfur; set the sum of the charges equal to zero since the compound is neutral. Follow the order of operations and use an inverse operation to isolate x. (+1)(2) + x + (-2)(4) = 0 Sum of the charges = 0. 2 + x - 8 = 0 Multiply from left to right. x - 6 = 0 Add.
Chad begins a chapter on Electrochemistry with a lesson on How to Assign Oxidation Numbers (i.e. Oxidation States). Six rules for determining oxidation numb...
The oxidation number of a monatomic ion equals the charge on the ion. The sum of oxidation numbers in a neutral compound is zero. The sum of all oxidation states in a polyatomic ion must equal the ...
7) The oxidation number of Group 1A elements is always +1 and the oxidation number of Group 2A elements is always +2. 8) The oxidation number of oxygen in most compounds is -2. 9) Oxidation numbers for other elements are usually determined by the number of electrons they need to gain or lose to attain the electron configuration of a noble gas.
Assigning Oxidation Numbers. Step 1: Assign any element that is not combined with any other elements an oxidation number of zero. Step 2: Assign any monatomic ion an oxidation number equal to the ...
Oxidation-reduction reactions, commonly known as redox reactions, are reactions that involve the transfer of electrons from one species to another. The species that loses electrons is said to be oxidized, while the species that gains electrons is said to be reduced. We can identify redox reactions using oxidation numbers, which are assigned ...
This chemistry video tutorial provides a basic introduction on how to calculate oxidation numbers. It discusses how to find the oxidation states of elements...
Assign an oxidation number of -2 to oxygen (with exceptions). In almost all cases, oxygen atoms have oxidation numbers of -2. There are a few exceptions to this rule: When oxygen is in its elemental state (O 2), its oxidation number is 0, as is the case for all elemental atoms. When oxygen is ...
Assigning oxidation numbers to organic compounds. The oxidation state of any chemically bonded carbon may be assigned by adding -1 for each bond to more electropositive atom (H, Na, Ca, B) and +1 for each bond to more electronegative atom (O, Cl, N, P), and 0 for each carbon atom bonded directly to the carbon of interest. ...